Simple Serial Dilution Calculation
In S:T format: dilution factor = T/S Diluting a sample twice This example explains the basic steps to use dilution factors forward and backward. Using them forward means finding the cell density after all the dilutions are performed, starting from the original solution.
Official Apple Support. Popular topics. Are you a new Mac user? We’re always happy to welcome a new Mac user. Here are a few topics to help you get started. Stylewriter 4 04 Keygen Torrent. Cracks, serial number generators, keygens. More than 400k cracks and serial key generators for any software. It gives recommendations and remedies progressively and with adaptable choices, simply duplicate content to the clipboard, the product will get your content. Stylewriter 4 04 keygen torrent.
Using them backwards means finding out the original cell density, starting from the most diluted one. Remember this is only an illustration example (it’s impossible you’ll have exactly 11 cells in a beaker and they will definitely not be this big). • We have 11 cells in a beaker suspended into 15mL of water. • We add 10mL of water to the beaker • We now have 11 cells in the beaker into 25mL of water.
• We add an extra 15mL of water to the beaker. • We end up with still 11 cells in the beaker but they’re suspended into 40mL of water. Let’s do the calculations forward: The cell density of 1 is 11 cells / 15mL water = 0.7333 cells/mL. We want to find the cell density of 3 without redoing the calculations with cells, and using the previously calculated cell density. The final volume is 25mL and the initial volume is 15mL, so the dilution factor is 25/15 = 1.6667 (keep all your trailing sixes for accuracy). We can now apply it to the original cell density: 0.73 / 1.6667 = 0.44 cells/mL; and we can check it using the original method: 11 cells / 25mL = 0.44 cells/mL.
Same thing for the dilution from 3 to 5: the cell density of 3 is 0.44 cells /mL. The dilution factor in this step is 40mL / 25mL = 1.6. We divide the cell density by the dilution factor and we get: 0.44 / 1.6 = 0.275 cells/mL. Double checking: 11 cells / 40mL = 0.275 cells/mL 🙂.
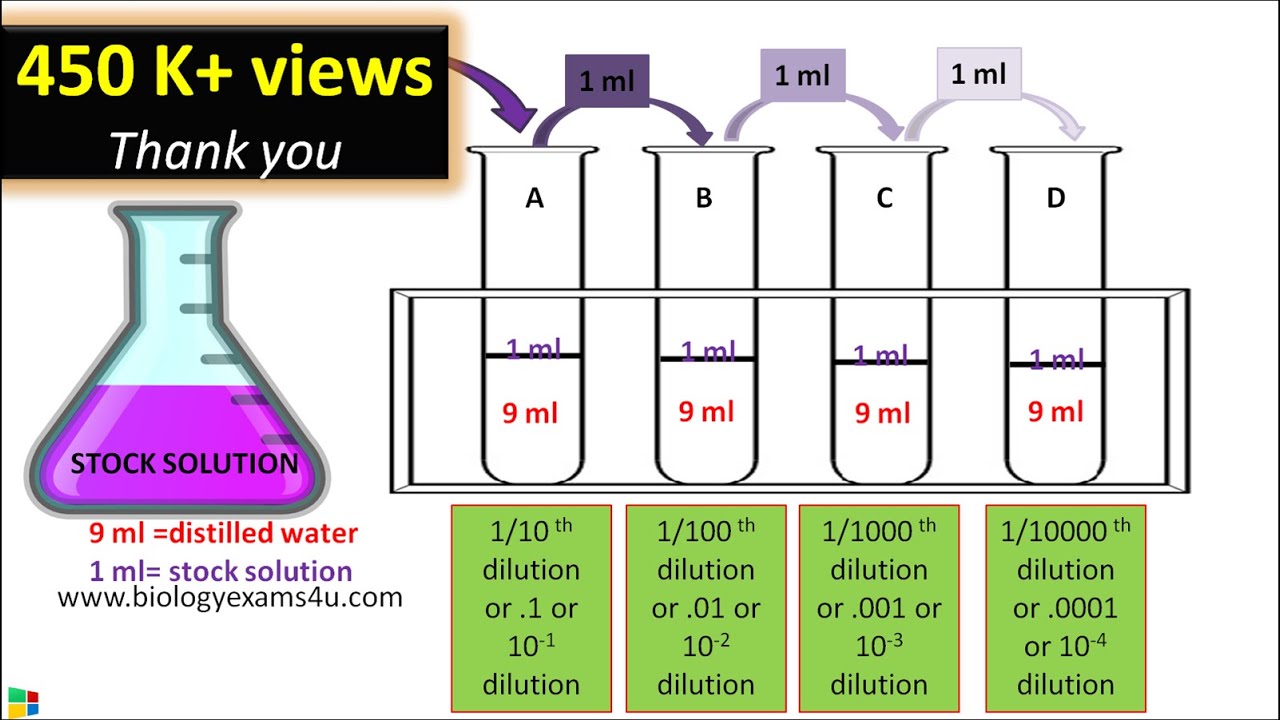
Explanation: A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. In serial dilutions, you multiply the dilution factors for each step. The dilution factor or the dilution is the initial volume divided by the final volume.
A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. In serial dilutions, you multiply the dilution factors for each step. The dilution factor or the dilution is the initial volume divided by the final volume. #DF = V_i/V_f# For example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution, #DF = V_i/V_f# = #(1'mL')/(10'mL') = 1/10#. This is a 1:10 dilution. Example 1 What is the dilution factor if you add 0.2 mL of a stock solution to 3.8 mL of diluent?
#V_f# = 0.2 mL + 3.8 mL = 4.0 mL #DF = V_i/V_f# = #(0.2'mL')/(4.0'mL') = 1/20#. This is a 1:20 dilution. Example 2 If you did the above dilution four times, what would be the final dilution factor? Solution 2 Remember that serial dilutions are always made by taking a set quantity of the initial dilution and adding it successively to tubes with the same volume.
So you multiply each successive dilution by the dilution factor. You would transfer 0.2 mL from Tube 1 to 3.8 mL of diluent in Tube 2 and mix.
Then transfer 0.2 mL from Tube 2 to 3.8 mL of diluent in Tube 3 and mix. Repeat the process until you have four tubes. The dilution factor after four dilutions is #DF = 1/20 × 1/20 × 1/20 × 1/20 = 1/160000# = 1:160 000 If the concentration of the original stock solution was 100 µg/µL, the concentration in Tube 4 would be 100 µg/µL × #1/160000# = 6.25 × 10⁻⁴ µg/µL Hope this helps.